The first successful “gene editing” of human embryos to prevent transmission of inherited disease, announced this week, is a landmark in biotechnology. Humanity has gained the power to engineer its own evolution by making genetic changes that will be passed down through future generations.
首例为了防止遗传疾病传播而对人类胚胎进行“基因编辑”的成功实验在本周宣布,这是生物技术领域的一块里程碑。人类获得了设计自身进化的力量——通过将会传给子孙后代的基因改变。
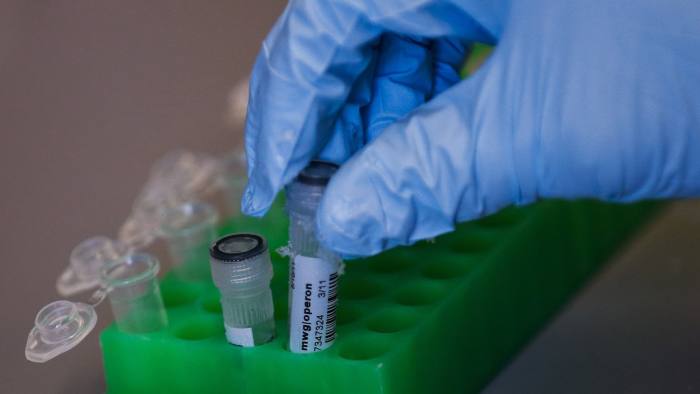
Most scientists have rightly greeted the achievement, by a US-based team working with colleagues in South Korea and China, as an experimental tour de force. Using new technology called Crispr, the researchers removed a genetic mutation that causes sudden heart failure from dozens of early human embryos with impressive precision and efficiency — and without the “off-target” impact on other genes that many feared would be an unwanted side-effect of gene editing. But much more work will be needed to assess the technique’s safety before anyone plans to implant an edited embryo into a womb.
多数科学家正确地欢迎由一个美国团队在与韩国和中国同事合作下取得的这项成就,视其为一个实验杰作。研究人员利用基因编辑技术CRISPR,以令人印象深刻的精确度和效率,从几十个早期阶段人类胚胎中切除了将会导致突发性心脏衰竭的基因突变,而且没有对其他基因造成“脱靶”损伤,而很多人曾经担心这种损伤将是基因编辑的一种不可取的副作用。但是,在任何人计划将编辑好的胚胎植入子宫之前,还需要进行大量工作,以评估这种基因编辑技术的安全性。
The project’s success should inspire governments, regulatory authorities and medical academies around the world to prepare more actively for clinical trials leading to genetically engineered babies. On top of thorough safety testing, extensive regulatory and ethical work with maximum public involvement will be needed before this can happen — building on the activities of bodies, such as the Nuffield Council on Bioethics in the UK and American Society of Human Genetics, that are already engaging with the subject.
这个项目的成功应该鼓舞世界各国政府、监管机构和医学院校更积极地准备最终将会带来“基因工程婴儿”的临床试验。在临床试验可以启动之前,除了严密的安全测试以外,还需要广泛的监管和伦理研究,并且让公众最大限度地参与讨论——以已经在研究这个主题的机构,如英国纳菲尔德生物伦理学理事会(Nuffield Council on Bioethics)和美国人类遗传学会(American Society of Human Genetics)的工作为基础。
If we dismiss the idea of an absolute religious or philosophical prohibition of any tampering with human evolution, even to prevent the most horrible diseases, then there are several other issues to consider.
如果我们拒绝以宗教或哲学为本的绝对禁止对人类进化作任何篡改(哪怕为了预防最可怕的疾病也不能例外)的观念,那么还有其他几个议题需要考虑。
The “slippery slope” argument — that technology developed for good medical reasons will inevitably be applied for ethically more dubious purposes such as producing “designer babies” with enhanced looks, athletic ability or intelligence — justifies strong regulatory controls to prevent such abuse, but is surely no reason to abandon research that aims to reduce human suffering.
“滑坡”论点——出于良好医学理由而开发的技术,将不可避免地被应用于在伦理上更为可疑的目的,例如生产具有增强外表、运动能力或智力的“设计师宝宝”——说明需要强有力的监管控制来防止这种滥用,但这肯定不是放弃旨在减少人类苦难的研究的理由。
In fact there are sound scientific reasons why it would be extremely hard to apply the technology to genetic enhancement. One is that the desired traits depend on many genes acting together, most of them unknown; these will lie far beyond the scope of DNA editing for the foreseeable future. Another is that the experiment published this week worked well because each embryo carrying a defective heart gene also had a healthy copy, which acted as a template for the DNA repair process. This should make Crispr editing possible in thousands of other inherited disorders in which sufferers have one good and one bad copy of the gene responsible. There would be no comparable template for genetic enhancement.
事实上,有充分的科学理由说明,为什么将这项技术应用于基因增强是极其困难的。其中一个原因是,想要的特性取决于许多基因的共同作用,其中大多数是未知的;在可预见的未来,这些将远远超出DNA编辑的范畴。另一个原因是,本周发表的实验之所以进行顺利,是因为每一个携带有缺陷心脏基因的胚胎都有一个健康的“副本”,后者可作为DNA修复过程的模板。这种方法应该使CRISPR编辑能够被应用于数千种其他遗传疾病——只要患者有一个好版本的基因,也有一个要负责的坏版本基因。对于基因增强,就没有这种可比较的模板了。
Critics also question the need for gene editing when pre-implantation diagnosis (PGD), which selects healthy IVF embryos by a DNA test, can do the job, too. This is often true, but sometimes no healthy embryos are available — and, even when they are, gene editing could increase the number available to implant in the womb.
当胚胎植入前遗传学诊断(PGD)——通过DNA测试来选择健康的体外受精(IVF)胚胎——也可以达到同样目的时,批评者还质疑基因编辑的必要性。这个命题往往是正确的,但有时没有健康的胚胎可用——再说即使存在健康的胚胎,基因编辑也可以增加可植入子宫内的胚胎数量。
A more practical barrier to embryonic gene editing may be cost and complexity. The Crispr procedure is bound to be expensive, even after streamlining for clinical use, and PGD will still be needed before implantation to ensure that the DNA has been repaired. The number of beneficiaries may be small in the early years, but the technology’s long-term promise is so great that society must develop a framework for its clinical development. Producing healthy babies is a laudable aim.
胚胎基因编辑的更实际障碍可能是成本和复杂性。CRISPR手术肯定是昂贵的,即使在经过精简用于临床之后也会如此,同时在植入胚胎前仍然需要PGD诊断,以确保DNA已被修复。受益者的数量在初期可能很少,但这种技术的长期前景是如此之大,社会必须为其临床开发制定一个框架。生产健康的宝宝是值得称道的目标。












